What You Need To Know About Galvanic Action
When two electro chemically dissimilar metals are in contact, galvanic action occurs. Here, ions and electrons move from one metal to the other. As the other metal’s ions are deposited, one metal corrodes. It is, therefore, crucial to insulate these metals which are dissimilar from each other. In this way, corrosion acceleration is prevented. More times than not, particularly where saltwater is concerned, this water serves – between the two metals – as their conductive path. So, it is obviously important, particularly in wet conditions, to keep dissimilar metals separated. Read on to find out more about galvanic action.
Anodes and Cathodes
- Anodes: Susceptible to corrosion because they are less stable than cathodes. Examples include aluminum, galvanized steel, and zinc.
- Cathodes: Stable or noble metals not usually corrosion prone. Examples are titanium, nickel, silver, and gold. Because they do not corrode rapidly, these materials are frequently used in jewelry.
It is essential to keep metals apart because cathodes and anodes will react with each other. As an example: If, with silver, zinc was to come in contact, and was in a solution of saltwater, the zinc would corrode because it’s ions would be transferred to the silver. Metals have a lesser tendency to corrode when, on the galvanic corrosion scale, they are close to each other. See below:
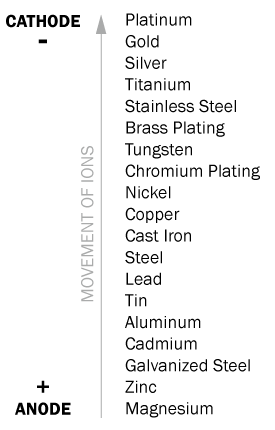
The Galvanic Series
In the galvanic series, the above scale displays the relative location of numerous metals. However, including the environment and the alloy of the metal, there are numerous factors that can affect corrosion resistance.
What Does The Scale Tell Us About Galvanic Action?
The scale above demonstrates the principles involved in how some metals react to each other. It is also only meant to be taken as a general outline as it is presumed the metals were submerged in seawater. Here is an example of one scenario:
Let’s say that galvanized zinc metal fasteners were used to keep an element of stainless-steel fastened in place. As zinc’s ions move in the direction of the stainless steel, the galvanized fasteners would corrode rapidly. This would, in turn, cause the element of stainless steel to come loose.
Engineers and architects, needless to say, must, at all times, be wary when choosing fasteners to be used on fabrications made from metal. This is exceedingly important in the presence of moisture.
Whom to Turn to for Thermal Spray Coatings for Galvanic Action?
Galvanic action, or cathodic delamination, in certain industries, causes separation of metals, components, etc. Again, this is abundantly apparent in industries that deal with saltwater (i.e., marine; defense and military; offshore drilling rigs, etc.). This can create catastrophic failures causing water to leak into places it should never, ever be!
By using non-conductive ceramic coatings plasma sprayed onto certain parts, components, etc., at the interface area, galvanic action can be significantly eliminated.
For more than 10 years, A&A Coatings has been an approved supplier of NCC (non-conductive coatings) with a dedicated team of professionals. This way, you’re assured to receive your product coated on time and with the industry’s most competitive pricing. Highly recognized for our non-conductive, high-quality coatings, we are a NAVSEA-S9320-AM-PRO-030 approved supplier. If you have questions about what part or component you need coated, we will be happy to answer any and all of your queries. Feel free to give us a call at 888-679-5460, or click here to contact us.



